4 Best Practices for Effective Medical Software Testing Compliance

Introduction
Navigating the complex landscape of healthcare software testing is essential for developers who aim to deliver safe and compliant applications. Stringent regulations, such as HIPAA and FDA guidelines, necessitate a thorough understanding of these requirements. This knowledge is not merely advantageous; it is crucial for enhancing patient safety and the quality of care.
However, the journey toward compliance presents numerous challenges. Developers encounter issues ranging from integration difficulties to data security concerns. To effectively address these obstacles, it is vital for developers to implement best practices that ensure their software not only meets regulatory standards but also distinguishes itself in a competitive market.
Understand Healthcare Software Testing Requirements
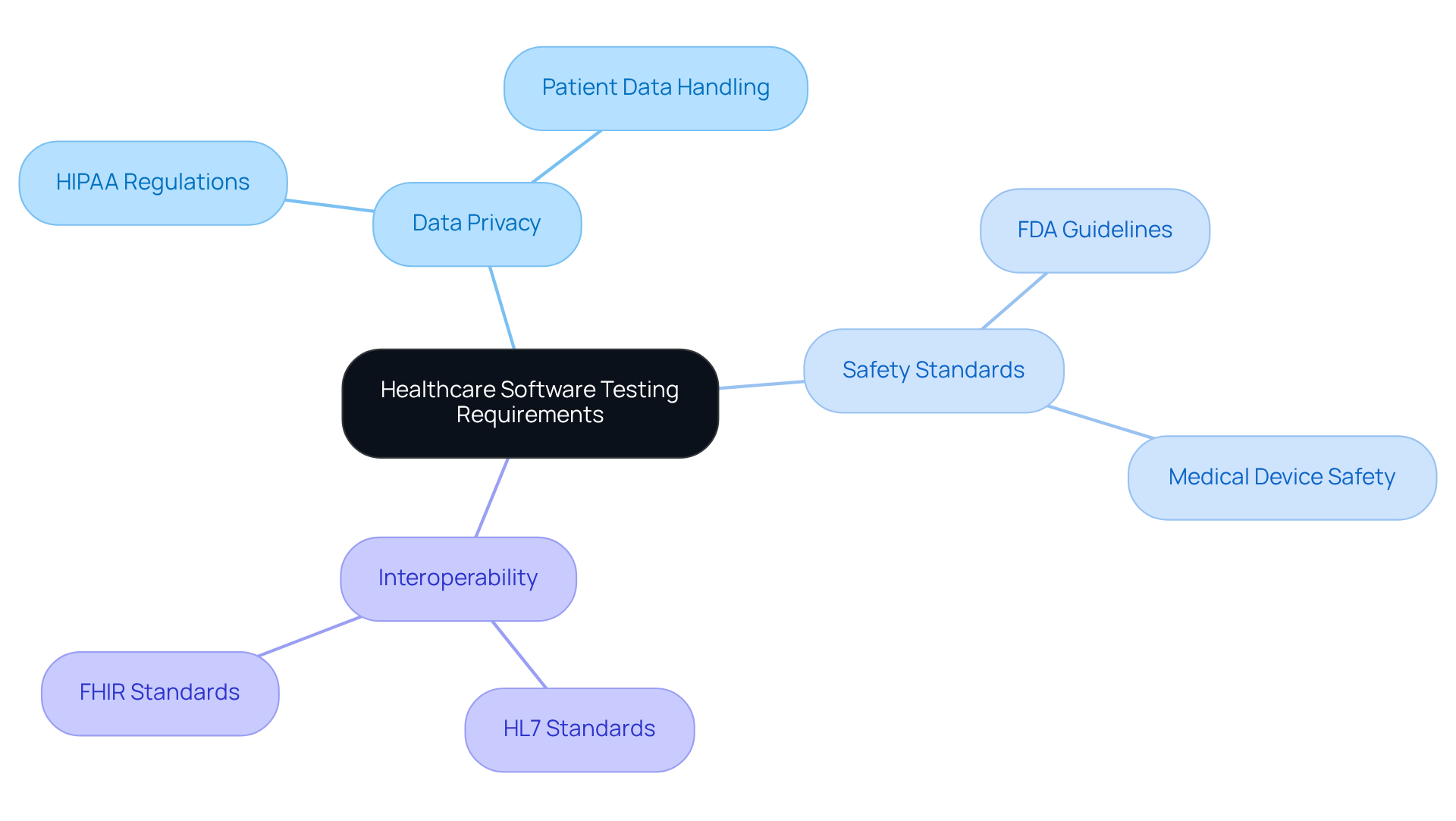
Healthcare applications must comply with various regulations, including HIPAA, FDA guidelines, and ISO standards. Understanding these requirements is essential for developers to ensure their applications meet legal and safety standards, particularly in the realm of medical software testing.
-
Data Privacy: It is imperative to handle patient data in accordance with HIPAA regulations, which impose strict controls over access and sharing of health information.
-
Safety Standards: Developers should familiarize themselves with FDA regulations governing medical devices and applications, ensuring that their products are safe for patient use.
-
Interoperability: Software must effectively communicate with other healthcare systems, adhering to standards such as HL7 and FHIR.
By thoroughly understanding these requirements, developers can engage in medical software testing to create applications that not only comply with regulatory standards but also enhance patient safety and the quality of care.
Explore Key Types of Healthcare Software Testing
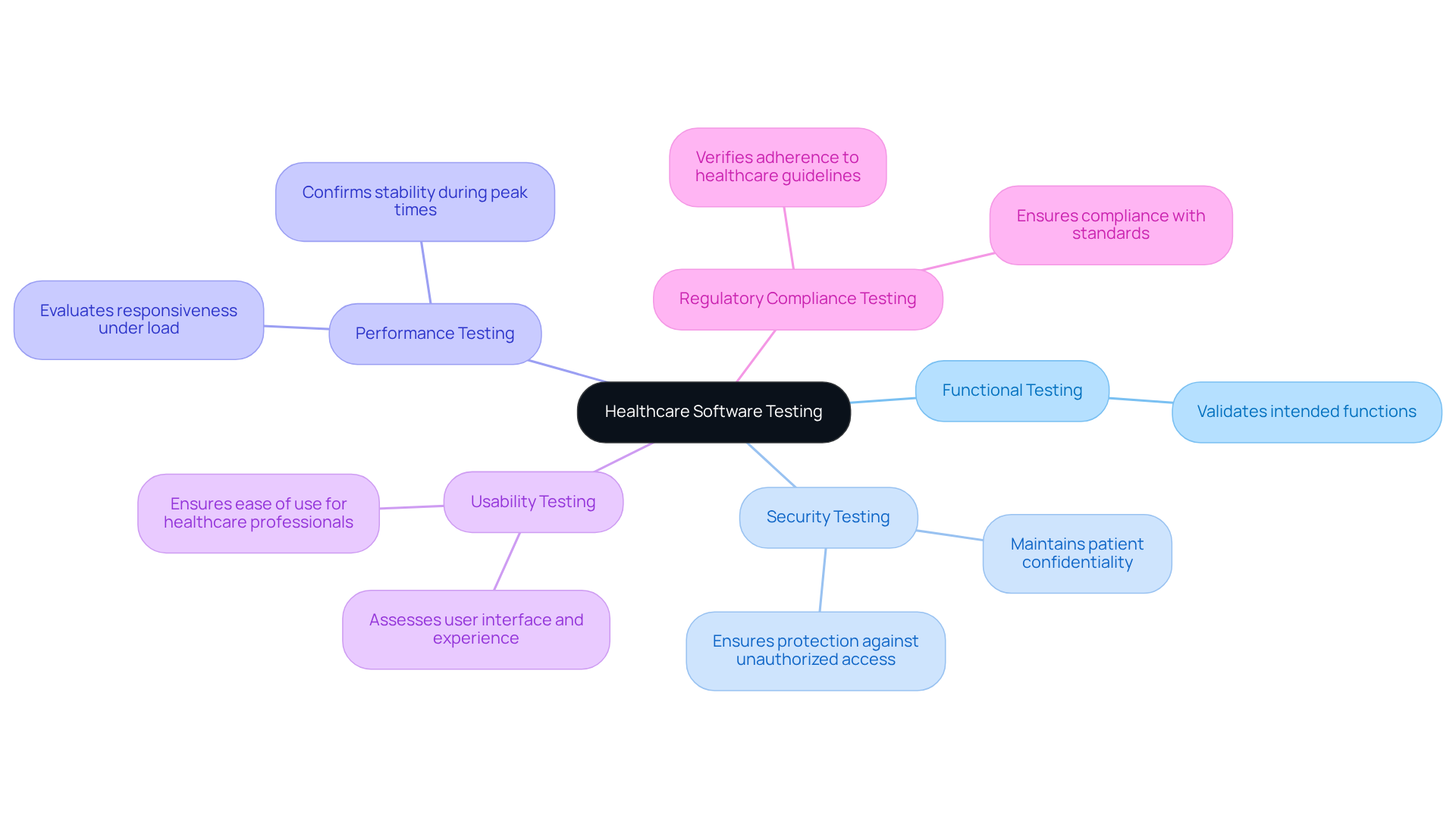
Healthcare application testing is a form of medical software testing that involves a variety of methodologies, each designed to address specific aspects of the system’s functionality and compliance. The primary types of testing include:
- Functional Testing: This type validates that the software performs its intended functions accurately.
- Security Testing: It ensures that the system is safeguarded against unauthorized access and data breaches, which is essential for maintaining patient confidentiality.
- Performance Testing: This evaluates the application’s responsiveness and stability under load, confirming its ability to handle high user volumes, especially during peak times.
- Usability Testing: This assesses the application’s user interface and experience, ensuring that healthcare professionals can effectively utilize it without extensive training.
- Regulatory Compliance Testing: It verifies that the application adheres to all relevant healthcare guidelines and standards.
By applying a combination of these evaluation types, organizations can ensure that healthcare applications are reliable, secure, and user-friendly.
Ensure Regulatory Compliance in Software Testing
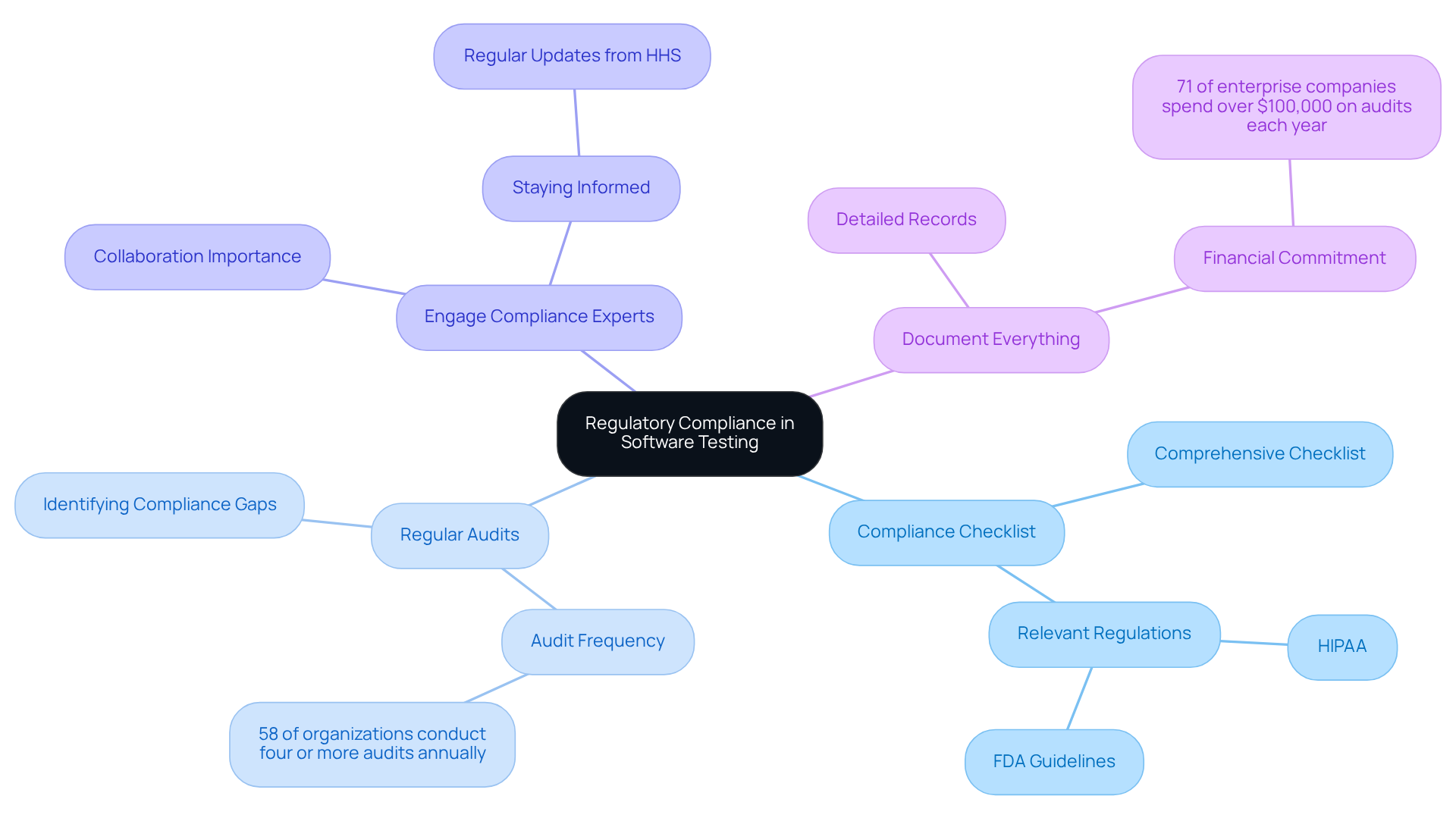
To ensure regulatory compliance in healthcare software testing, developers should adopt several best practices:
- Develop a Compliance Checklist: A comprehensive checklist should be created, encompassing all relevant regulations, such as HIPAA and FDA guidelines. This checklist must be utilized at each evaluation phase to ensure that all requirements are systematically addressed.
- Conduct Regular Audits: A schedule for periodic audits of both the system and testing processes should be implemented. These audits are essential for identifying compliance gaps and rectifying them promptly, thereby enhancing overall system integrity. In fact, 58% of organizations conducted four or more audits annually, underscoring the importance of this practice in maintaining regulatory adherence.
- Engage Compliance Experts: Collaboration with regulatory experts is crucial to remain informed about evolving healthcare regulations. Their insights can be invaluable in integrating necessary changes into the evaluation process, ensuring that the software remains compliant. As Philip LaRocca notes, “Organizations need to stay current on changes affecting their practice due to regular updates from the Department of HHS.”
- Document Everything: Detailed records of examination methods, outcomes, and verification checks must be kept. This documentation acts as vital proof of adherence to regulations during audits, reinforcing the organization’s dedication to following rules. Notably, 71% of enterprise companies spend over $100,000 on audits each year, highlighting the financial commitment to thorough documentation.
By incorporating adherence evaluations into the medical software testing assessment process, developers can significantly reduce risks and guarantee that their applications fulfill the required legal criteria. Additionally, 66% of organizations are expected to use purpose-built technology to manage compliance risk in 2025, emphasizing the technological aspect of compliance management.
Identify and Overcome Common Testing Challenges
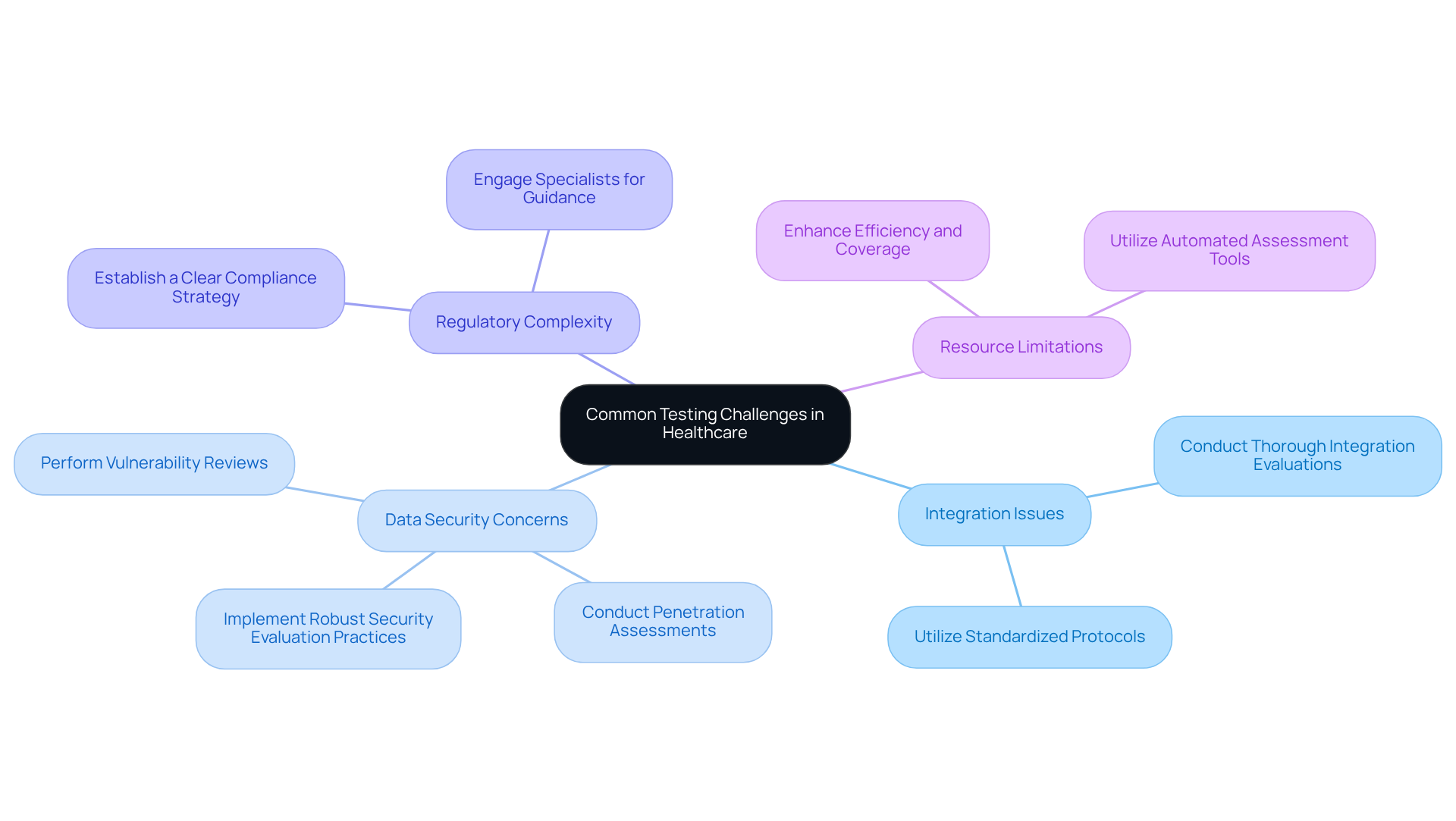
Evaluating healthcare applications presents distinct challenges that can hinder the development process. These challenges include:
- Integration Issues: Healthcare software frequently requires integration with existing systems, which can result in compatibility problems. To address this, it is essential to conduct thorough integration evaluations and utilize standardized protocols.
- Data Security Concerns: Safeguarding sensitive patient data is critical. Implementing robust security evaluation practices, such as penetration assessments and vulnerability reviews, is necessary to identify and mitigate potential risks.
- Regulatory Complexity: The multitude of regulations can be overwhelming. Establishing a clear compliance strategy and engaging specialists to guide the evaluation process is advisable.
- Resource Limitations: Limited evaluation resources can impact the quality of assessments. Utilizing automated assessment tools can enhance efficiency and coverage.
By proactively identifying these challenges and implementing effective solutions, developers can facilitate a smoother medical software testing process and deliver high-quality healthcare software.
Conclusion
Understanding and implementing effective practices for medical software testing compliance is crucial for developers in the healthcare sector. By prioritizing regulatory adherence, organizations can ensure that their applications not only meet legal standards but also enhance patient safety and care quality. This commitment to compliance is fundamental in navigating the complexities of healthcare software development.
The article highlights essential practices such as:
- Understanding healthcare software testing requirements
- Exploring various testing methodologies
- Ensuring regulatory compliance
- Addressing common challenges
Key insights include:
- The importance of developing comprehensive compliance checklists
- Conducting regular audits
- Engaging compliance experts
- Meticulously documenting processes
Additionally, recognizing and overcoming integration issues, data security concerns, and resource limitations is vital for successful software testing in the healthcare environment.
Ultimately, the significance of rigorous medical software testing compliance cannot be overstated. As the landscape of healthcare regulations continues to evolve, staying informed and proactive is essential. Developers and organizations are encouraged to adopt these best practices and leverage technology to enhance compliance management. By doing so, they not only protect patient data and ensure safety but also contribute to the overall advancement of healthcare technology.
Frequently Asked Questions
What regulations must healthcare applications comply with?
Healthcare applications must comply with various regulations, including HIPAA, FDA guidelines, and ISO standards.
Why is it important for developers to understand healthcare software testing requirements?
Understanding these requirements is essential for developers to ensure their applications meet legal and safety standards, particularly in the realm of medical software testing.
What does HIPAA regulate in healthcare software?
HIPAA regulates the handling of patient data, imposing strict controls over access and sharing of health information.
What safety standards should developers be familiar with?
Developers should familiarize themselves with FDA regulations governing medical devices and applications to ensure their products are safe for patient use.
What is interoperability in healthcare software?
Interoperability refers to the ability of software to effectively communicate with other healthcare systems, adhering to standards such as HL7 and FHIR.
How can understanding healthcare software testing requirements benefit patient safety?
By thoroughly understanding these requirements, developers can engage in medical software testing to create applications that comply with regulatory standards, thereby enhancing patient safety and the quality of care.